Background: We have previously published that pancreatic cancers over-express an immunosuppressive peptide, vasoactive intestinal polypeptide (VIP), and that a novel combination of antin-PD1 antibodies and small molecule antagonists to the VIP receptor can induce complete remission of pancreatic ductal adenocarcinoma (PDAC) in multiple mouse models (Ravibndranathan, Nat. Comm. 2022). To enhance the efficacy of this novel immunotherapy approach, we hypothesized that infusions of additional donor T cells to PDAC-bearing mice receiving the combination of anti-PD1/VIP receptor antagonists would lead to more intra-tumoral effector T cells and increased tumor regression rates. To investigate the potential impact of adoptive splenocyte infusion on checkpoint immunotherapy, we established sub-cutaneous KPC PDAC tumors in C57Bl/6 mice. As previously described, we treated them with a combination of ANT308 (VIP-receptor antagonists) and anti-PD1. We tested whether adding splenocyte infusions from donor mice that were immunologically naïve or donor mice previously engrafted with PDAC with regressed (positive control) or progressed tumors (negative control) would enhance the efficacy of the immune checkpoint blockade therapy.
Methods: C57BL/6 B6 CD45.1 recipient mice were inoculated subcutaneously with 1 x 10 6 KPC-luc PDAC cell line cells on day -5. Recipient mice received anti-PD1 (200mg) every three days (totaling four doses) and 21 daily s.c. injections of 20 ug ANT308. Splenocytes from three groups of CD45.2+ C57BL/6 donor mice were injected into tumor-bearing recipients: naive mice, mice with a history of KPC regression post-ANT308/anti-PD1 treatment, and mice with progressed KPC tumors without any treatment. Anti-PD1 and ANT308 treatments were initiated simultaneously with adoptive splenocyte infusion (25 x 10 6 cells) on day 0. Tumor size was measured biweekly, and IVIS imaging was performed weekly. Blood was drawn weekly from recipient mice for flow cytometric analysis of immune cell subsets. A fraction of tumor-bearing recipient mice was euthanized ten days after donor splenocyte infusion and initiation of immune checkpoint therapy or at IAUCAC-defined endpoints. When tumor-bearing mice were euthanized, the spleen, lymph nodes, and tumors were harvested for further flow cytometric analysis. The remaining mice were monitored for tumor growth and survival kinetics.
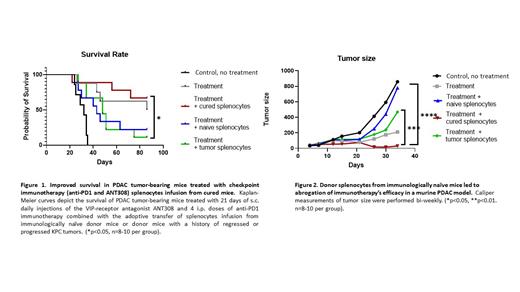
Results: Contrary to our original hypothesis, we observed substantially lower survival rates and faster tumor growth in the group that received splenocytes from immunologically naive donor mice versus the group treated with splenocytes from tumor-bearing mice (Figures 1A and 1B). Splenocytes from immunologically naive donors contained fewer Treg cells, whereas myeloid-derived suppressor cells (CD11b+Ly-6G+ Gr-1+) were increased in donor splenocytes from tumor-bearing mice. We next repeated the experiment utilizing the adoptive transfer of splenocytes from immunologically naïve donors separated into 8 x 10 6 MACS-purified T cell or 17 x 10 6 non-T cell fractions. Remarkably, the transfer of 8 x 10 6 immunologically naïve T cells abrogated the efficacy of immune checkpoint therapy leading to rapid tumor growth compared to tumor regression in mice treated with immune checkpoint therapy without added donor splenocytes or mice that received immune checkpoint therapy with 17 x 10 6 T-cell-depleted donor splenocytes. Flow cytometric and gene expression analyses of splenocytes from the recipient mice, and those not receiving donor T cell infusions, revealed that the tetramer population in the infusion group was significantly higher than in the group treated only with immune checkpoint inhibitors.
Conclusion: Our findings indicated that natural suppressor/ Treg in immunologically naïve splenocytes could exert a profound negative regulatory effect on immune checkpoint therapy's efficacy in a murine PDAC model. Despite having fewer Treg than splenocytes from tumor-bearing donors, immunologically naïve natural Treg had increased potency in suppressing anti-cancer immunity. These findings suggest the importance of the depletion of natural Treg in adoptive cell therapy strategies to treat solid tumor malignancies. This research underscores the critical role of immune contexture in shaping therapeutic outcomes and proposes a novel biomarker for patient stratification in PDAC immunotherapy.
Disclosures
Waller:Verastem: Consultancy, Research Funding; Allovir: Consultancy; CRISPR: Consultancy; Novartis: Consultancy, Research Funding; CSL Behring: Consultancy, Research Funding; ORCA: Research Funding; BMS: Research Funding; Sanofi: Research Funding; NCI R01: Research Funding; Secura: Research Funding; PartnersTherapeutics: Research Funding; Cambium Medical Technologies: Current equity holder in private company, Other: Founder; Cambium Oncology: Current equity holder in private company, Other: Founder.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal